Patient case #10 - Acute Myeloid Leukemia with NPM1 and FLT3-IDT mutation
Description
A 53-year-old male presented to the emergency department with acute onset of shortness of breath, tachycardia, and fever. He reported overwhelming fatigue, cutaneous rashes, and bone pain persisting for several weeks prior to admission. Given the acute presentation and systemic manifestations, he was immediately hospitalized for diagnostic evaluation.
CBC results:
| Test | Results | Unit |
|---|---|---|
| WBC | 165 | x109/L |
| HGB | 112 | g/L |
| MCV | 81.3 | fL |
| PLT | 18 | x109/L |
Smear analysis on CellaVision® DC-1:
| WBC differential | % | x109/L |
|---|---|---|
| Neutrophils | 7,6 | 12,6 |
| Lymphocytes | 0,8 | 1,5 |
| Monocytes | 1,7 | 2,8 |
| Myelocyte | 0,8 | 1,4 |
| Metamyelocyte | 1,7 | 2,8 |
| Blast | 87,3 | 144 |
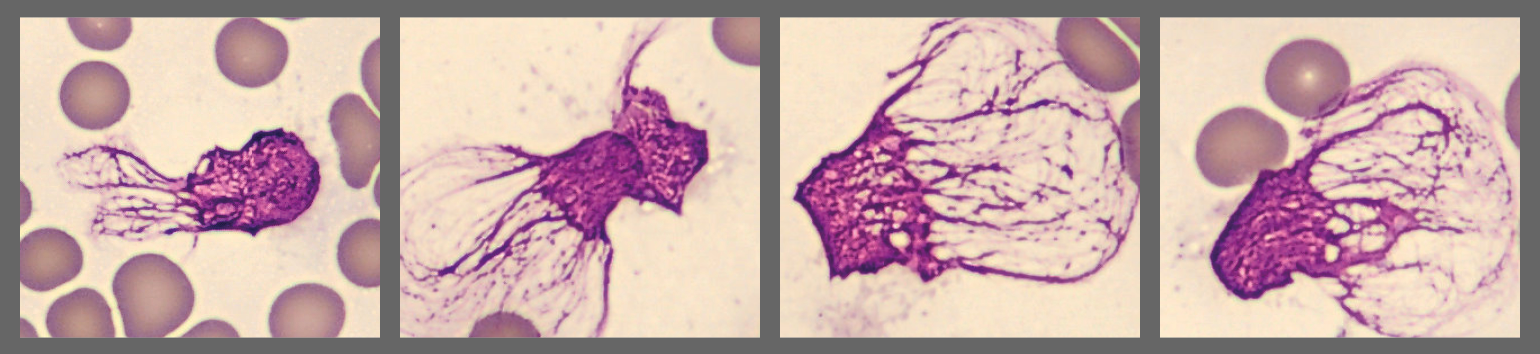
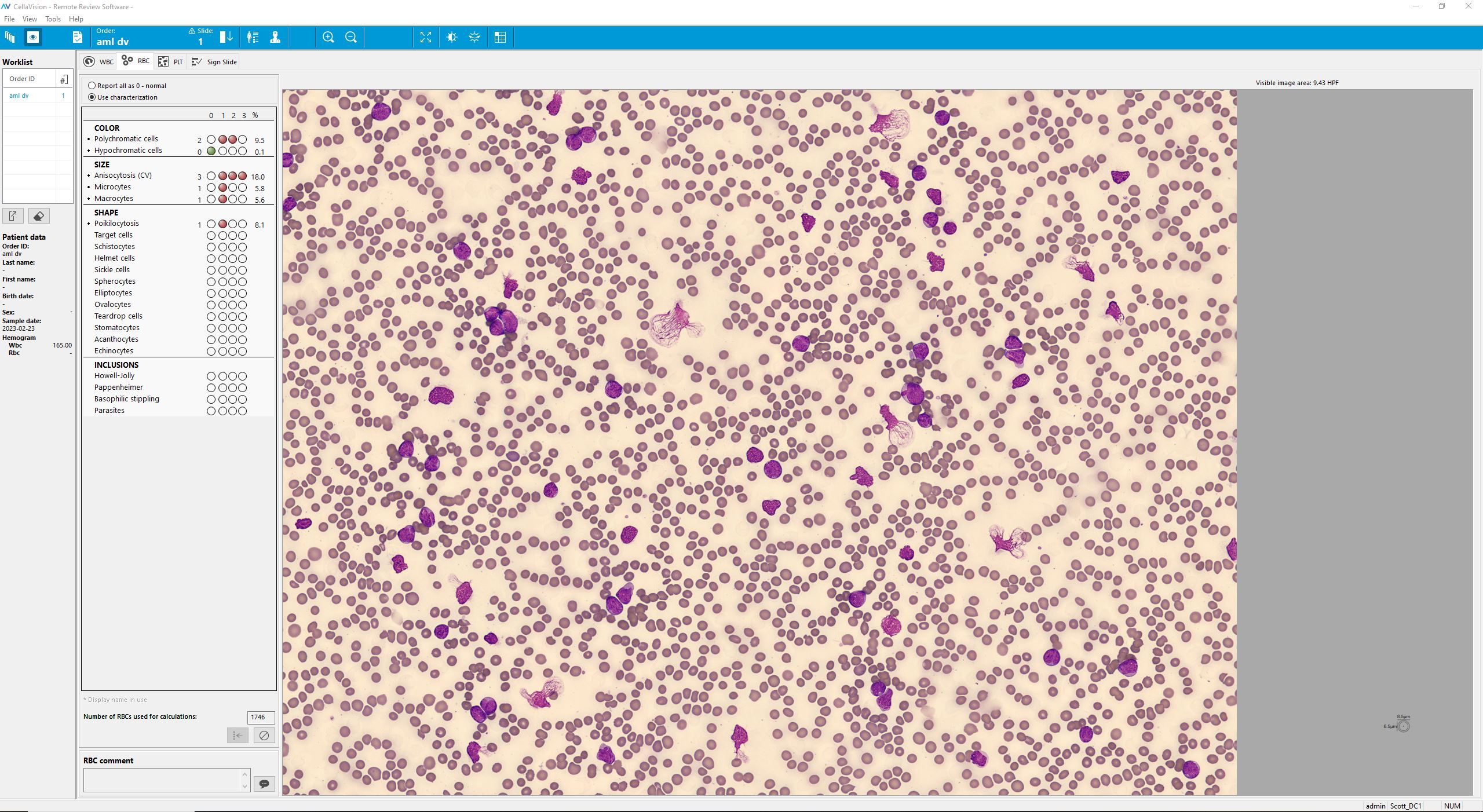
Microscopic analysis of the peripheral blood smear revealed a very high proportion of blast cells, heterogeneous in size and with irregular nuclear contours. Several smudge cells presented with a morphology showing dendritic projections which have recently been suggested to represent neutrophil extracellular traps (NETs).[1]
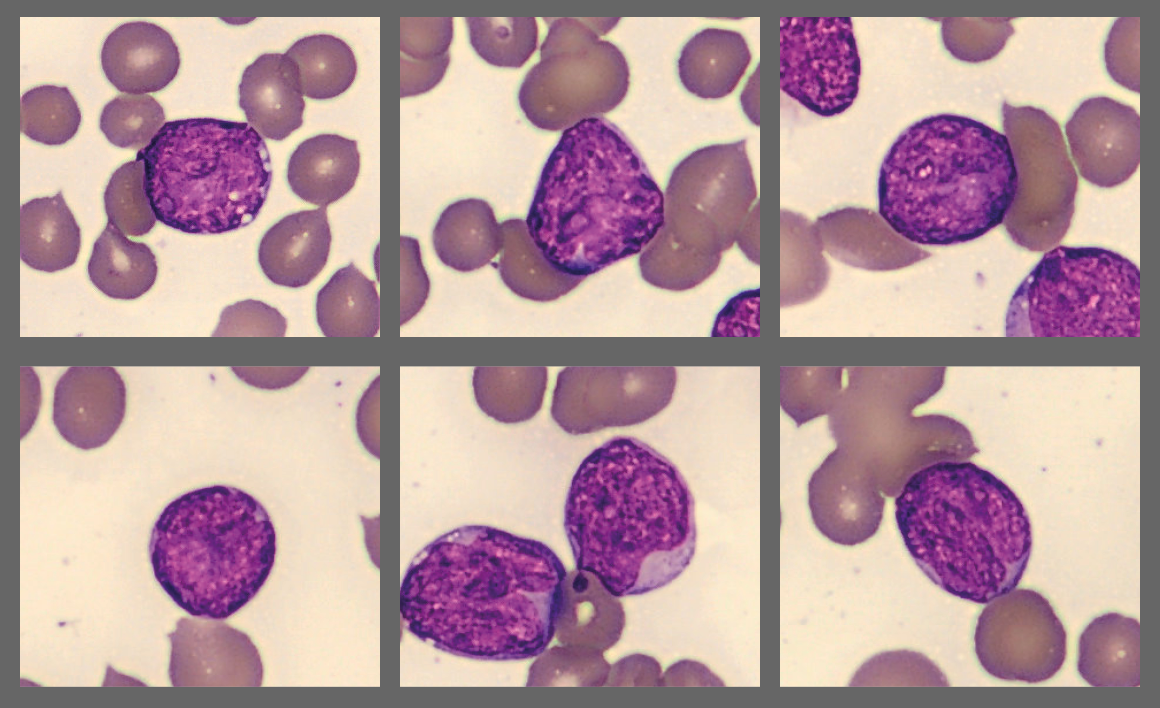
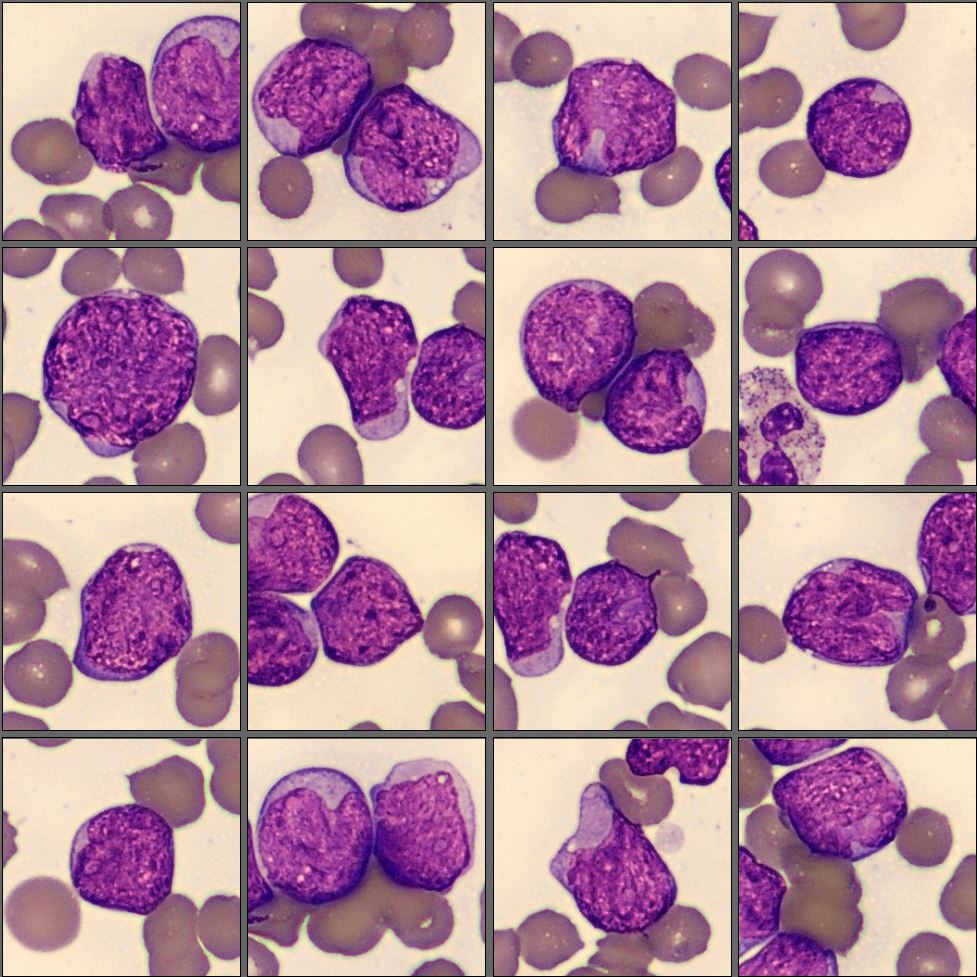
Flow cytometry results: CD2-, CD4- CD19-, CD56-, CD14-, CD11b+, CD13+, CD15+, CD65+, CD14-, CD64-, CD33+, dim CD34, CD117-, HLA-DR+, MPO+
Diagnosis
Acute Myeloid Leukemia (AML) with mutated NPM1 and FLT3-ITD
Discussion
Acute myeloid leukemia (AML) with NPM1 and FLT3-ITD mutations represents a biologically and clinically distinct subgroup. The NPM1 mutation leads to cytoplasmic mislocalization of nucleophosmin, affecting genomic stability and transcriptional regulation [2]. Concurrent FLT3-ITD mutations result in constitutive receptor activation, promoting uncontrolled proliferation and survival of leukemic blasts [3].
The prognostic implications of these mutations are complex. NPM1 mutation alone is generally associated with favourable outcomes in patients with a normal karyotype, whereas FLT3-ITD predicts higher relapse risk and poorer survival [3–5]. Large population-based studies have demonstrated that these effects are age-dependent: FLT3-ITD has the strongest adverse impact in younger patients (<60 years), whereas NPM1 mutations confer survival benefit primarily in older adults (60–74 years). In patients carrying both mutations, prognosis tends to be intermediate and less influenced by age [6].
The detection of neutrophil extracellular traps (NETs) in this patient’s smear is particularly noteworthy. Beyond their antimicrobial role, NETs are increasingly recognized as contributors to cancer progression, immune evasion, and treatment resistance [7,8]. Recent work has also established NET-related gene signatures as prognostic markers in AML, with potential to inform patient stratification and therapy [8]. Furthermore, targeting NETs through DNase treatment, PAD4 inhibition, or other strategies has been proposed as a novel therapeutic avenue in hematologic malignancies [7].
Sex also plays a modifying role: NPM1 mutations occur more frequently in females, whereas FLT3-ITD is more common in younger males. Prognostic effects also differ, with FLT3-ITD showing stronger negative influence in women and younger patients, while NPM1’s positive effect is most pronounced in older men [6].
From a therapeutic perspective, the European LeukemiaNet (ELN) recommendations incorporate both FLT3 and NPM1 status into risk stratification [9]. Patients with NPM1 mutation and no FLT3-ITD are classified as favourable risk, while those with concurrent FLT3-ITD (particularly at high allelic ratio) fall into intermediate or adverse categories [9]. Treatment strategies increasingly combine FLT3 inhibitors (such as midostaurin or quizartinib) with standard chemotherapy to improve outcomes in FLT3-ITD-positive AML [4]. In addition, NPM1 mutations serve as highly sensitive markers for measurable residual disease (MRD), supporting consolidation and transplant decisions [9].
Conclusion
This case exemplifies the power of integrating digital morphology using CellaVision DC-1 with molecular diagnostics (NPM1/FLT3 status) and population-based evidence to provide a nuanced prognosis. The ability of CellaVision to rapidly highlight blast populations and even visualize neutrophil extracellular traps (NETs) demonstrates its value in supporting early recognition of acute myeloid leukemia. By combining automated image analysis with expert review, CellaVision strengthens diagnostic accuracy, accelerates decision-making, and enhances the link between laboratory findings and individualized patient care.
___
Reference:
- Fedorov K, Barouqa M, Yin D, Kushnir M, Billett HH, Reyes Gil M. Identifying neutrophil extracellular traps (NETs) in blood samples using peripheral smear autoanalyzers. Life. 2023;13(3):623. doi:10.3390/life13030623
- Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352(3):254–66.
- Chauhan PS, Ihsan R, Singh LC, Gupta DK, Mittal V, Kapur S. Mutation of NPM1 and FLT3 genes in acute myeloid leukemia and their association with clinical and immunophenotypic features. Dis Markers. 2013;35(5):581–8.
- Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909–18.
- Port M, Böttcher M, Thol F, Ganser A, Schlenk RF. Prognostic significance of FLT3 internal tandem duplication, nucleophosmin 1, and CEBPA gene mutations for acute myeloid leukemia patients with normal karyotype and younger than 60 years: a systematic review and meta-analysis. Ann Hematol. 2014;93(8):1279–86.
- Juliusson G, Jädersten M, Deneberg S, Lehmann S, Mollgård L, Wennström L, et al. The prognostic impact of FLT3-ITD and NPM1 mutation in adult AML is age-dependent in the population-based setting. Blood Adv. 2020;4(6):1094–101.
- Cedervall J, Olsson AK. Targeting neutrophil extracellular traps: a novel strategy in hematologic malignancies. Front Immunol. 2024;15:1418256.
- Wang J, Wang H, Ding Y, Jiao X, Zhu J, Zhai Z. NET-related gene signature for predicting AML prognosis. Sci Rep. 2024;14:9115.
- Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47.