Monthly Cell Challenge #9 / 2025

The Case of Conflicting Clues
Description:
A 68-year-old woman presented to the emergency department with complaints of back and hip pain, progressive weight loss, shortness of breath, fever, generalized weakness, nausea, and vomiting.
Initial evaluation included a CBC with differential, together with additional laboratory investigations.
CBC results:
| Test | Result | Units |
|---|---|---|
| WBC | 14,5 | x109/L |
| HGB | 69 | g/L |
| MCV | 80 | fL |
| PLT | 81 | x109/L |
| NRBC's | ↑ | |
Initial laboratory evaluation showed leukocytosis, anemia, thrombocytopenia, and nucleated red blood cells (NRBCs). Other findings included elevated LDH, hypercalcemia, increased creatinine, and raised liver enzymes (AST/ALT), along with a high ESR. Albumin was within the normal range.
The automated cell counter flagged an abnormal result, leading to a manual differential and peripheral smear.
Smear preparation was difficult, and the stained slide appeared suboptimal, showing an unusual blue discoloration.
Smear review on CellaVision® DC-1
| WBC Differential | % | x109/L |
|---|---|---|
| Band neutrophils | 9 | 1,3 |
| Neutrophils | 42 | 6,1 |
| Eosinophils | 8,0 | 0,3 |
| Basophils | 3 | 0,4 |
| Lymphocytes | 12 | 1,7 |
| Monocytes | 12 | 1,7 |
| Promyelocytes | 2 | 0,3 |
| Myelocytes | 6 | 0,9 |
| Metamyelocytes | 3 | 0,4 |
| Blasts | 4 | 0,6 |
| Plasma cells | 6 | 0,9 |
| NRBC | 53/100 WBC | |
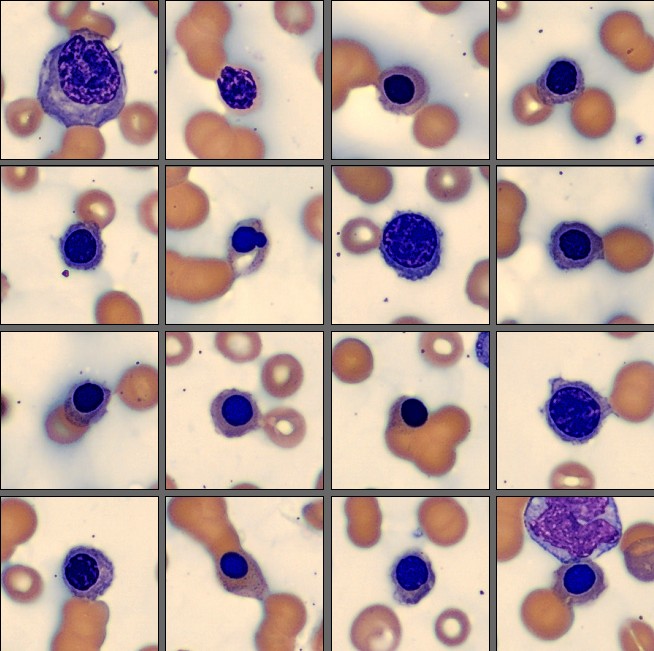
On the CellaVision® Remote Review Software, the Medical Technologist observed clumped red blood cells in the background of the white cell view. Red cell morphology demonstrated anemia with rouleaux formation, occasional target cells and stomatocytes, accompanied by a diffuse blue sheen across the blood film.
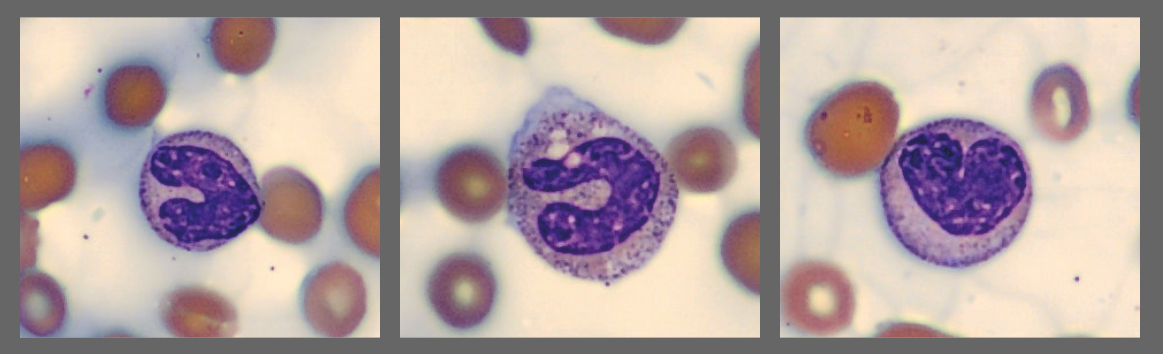
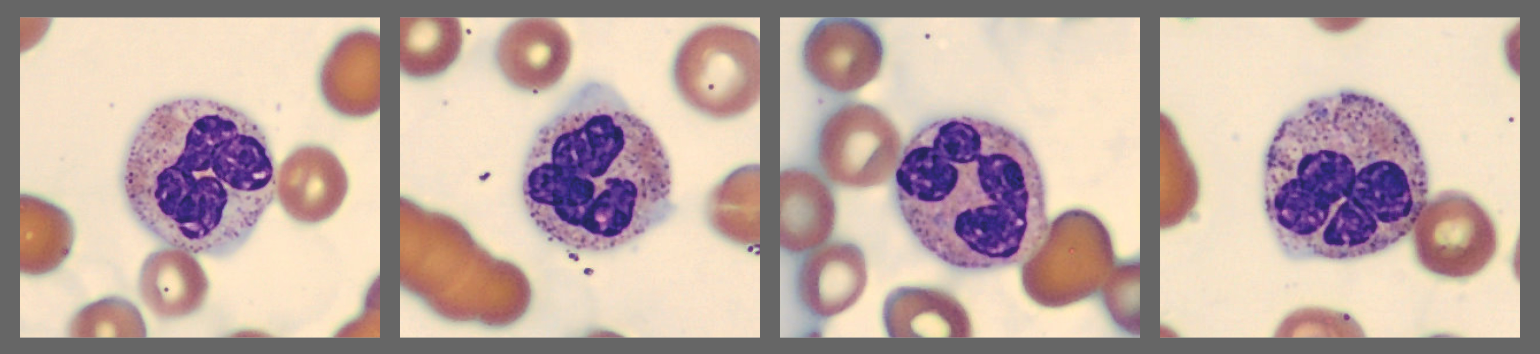
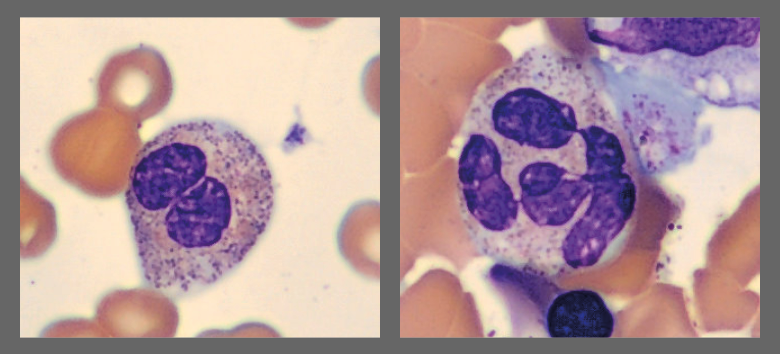
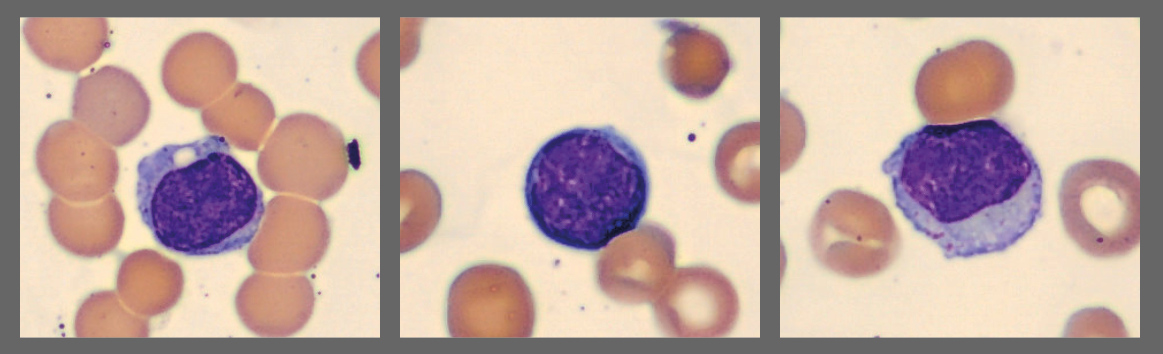
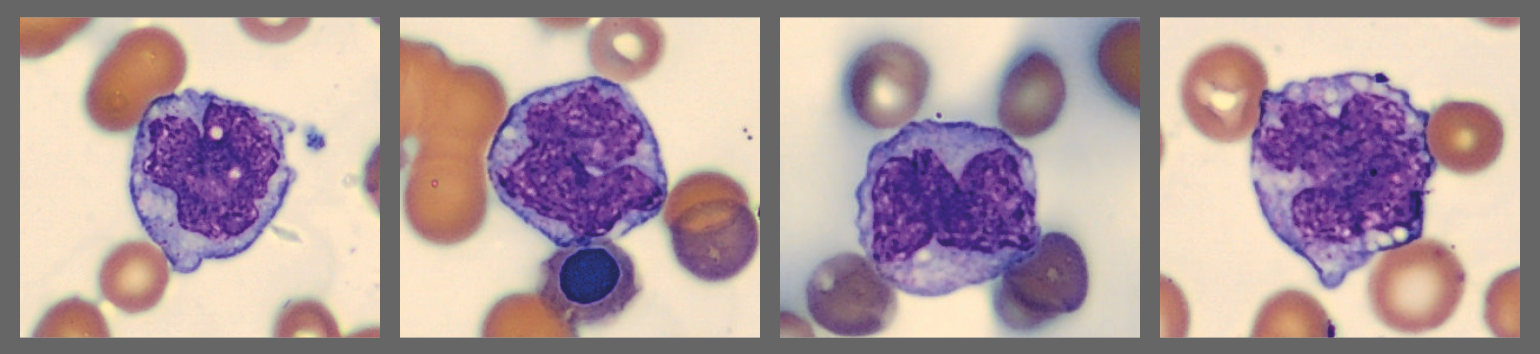
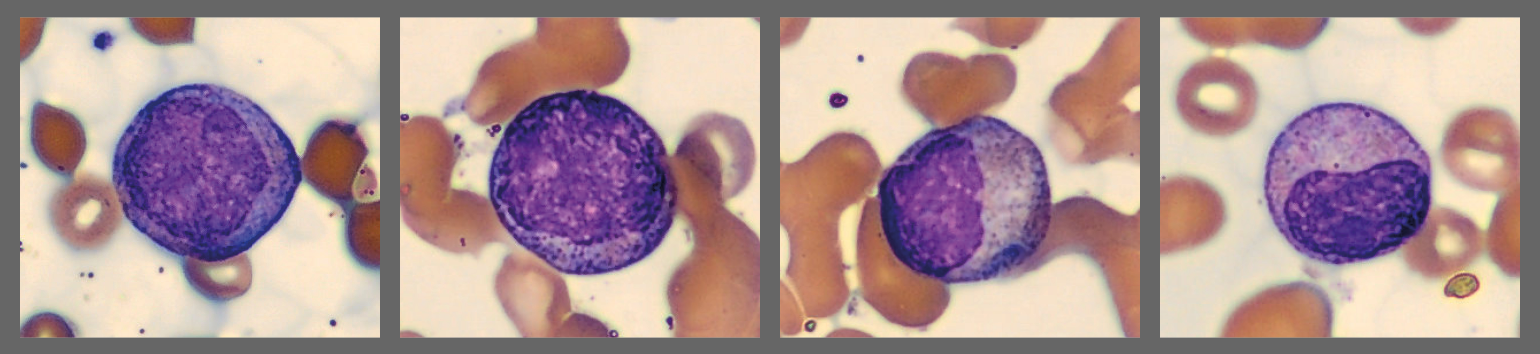
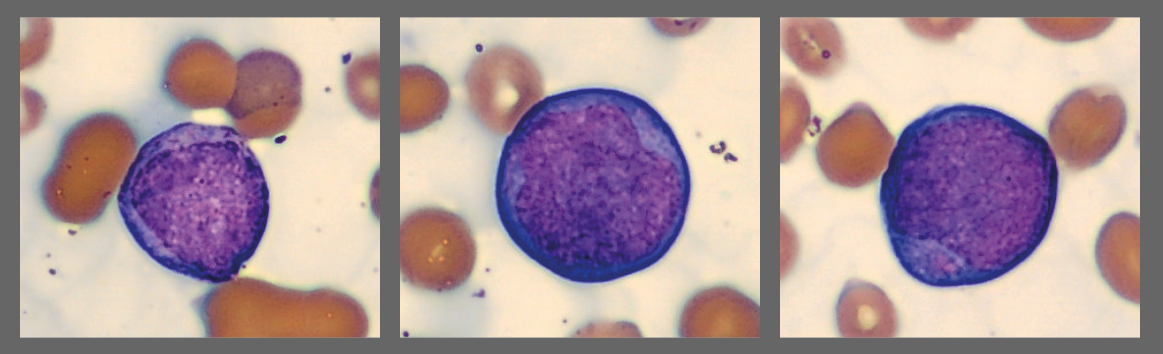
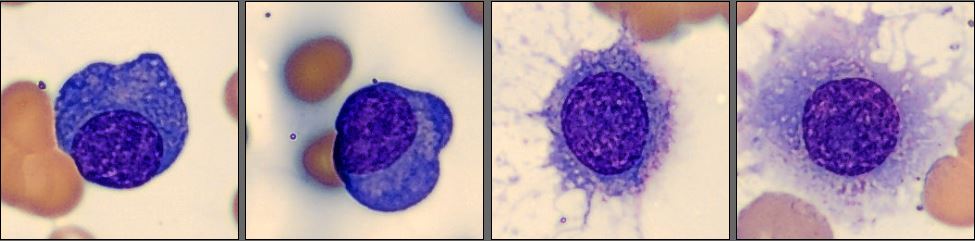
Among the white blood cells, some neutrophils showed dysplastic changes, including the presence of Pelgeroid forms, toxic granulation, and occasional hypersegmentation. A left shift was evident with representation of the full spectrum of immature granulocytes. Blast cells were identified at approximately 4 %, and plasma cells were also present at about 6 %.
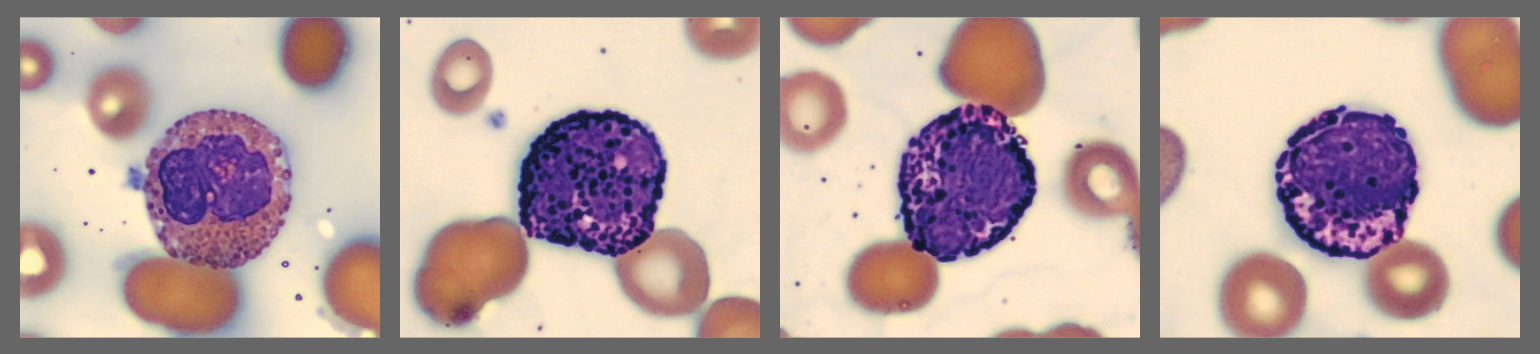
In addition, the smear demonstrated a markedly elevated number of nucleated red blood cells, some of which displayed dysplastic features.
Flow cytometry results: CD9+, CD10+, CD13+, CD19+, CD20+, CD33+, CD38+, CD138+, HLA-DR+, dim CD45, CD34+, CD117+, CD56+, and CD7+.
Cytogenetic analysis revealed a deletion of chromosome 5q (del[5q]).
Bone marrow examination showed 75% infiltration by lambda-restricted plasma cells.
Serum studies revealed markedly elevated immunoglobulins with IgG at 39.5 g/L and increased lambda free light chains at 4.72 g/dL.
Protein electrophoresis confirmed a monoclonal IgG lambda spike on serum protein electrophoresis (SPEP) and a monoclonal lambda chain on urine protein electrophoresis.
Diagnosis:
Multiple Myeloma with coexistence of MDS
Discussion:
This case illustrates a rare and diagnostically challenging scenario of dual hematologic malignancies: Multiple Myeloma (MM) with concurrent Myelodysplastic Syndrome (MDS) [1,2].
Multiple Myeloma (MM) is characterized by clonal plasma cell proliferation and commonly presents with anemia, bone pain due to lytic lesions, renal impairment, hypercalcemia, and monoclonal gammopathy. These features were evident in this patient through severe anemia, skeletal pain, renal dysfunction, and the presence of a monoclonal IgG lambda spike [1].
Myelodysplastic Syndrome (MDS) is a clonal stem cell disorder that causes ineffective hematopoiesis, cytopenias, and dysplasia across hematopoietic lineages. This patient showed typical findings, including abnormal NRBCs, dysplastic neutrophils, and immature myeloid precursors. The cytogenetic abnormality del(5q) further reinforced the diagnosis and is a well-established prognostic marker within MDS risk stratification systems [3].
Although uncommon, the coexistence of MM and MDS has been reported, particularly in older patients and in those with prior chemotherapy or stem cell transplantation. Overlapping clinical features such as anemia can complicate diagnosis, highlighting the importance of integrating morphology, cytogenetics, and flow cytometry for accurate classification [2,3].
Therapeutic considerations:
Managing MM with concurrent MDS is challenging, as treatments for one condition can worsen the other. Lenalidomide is notable because it is a guideline-supported agent in MM regimens and has proven activity in del(5q) MDS, offering potential dual benefit in carefully selected cases. In del(5q) MDS, landmark studies demonstrated substantial hematologic and cytogenetic responses to lenalidomide; mechanistic work shows it targets CK1α, providing a biologic rationale for its efficacy [1,4,5]. Close monitoring is essential to avoid exacerbating cytopenias [1,4].
Prognosis:
Coexistent MM/MDS is associated with poorer outcomes than either disease alone in small series and reports, largely due to compounded cytopenias, infection risk, and treatment constraints. While lenalidomide can produce meaningful hematologic improvements in del(5q) MDS, overall management remains complex and must be individualized [2,4,5].
References:
[1] Dimopoulos MA, Moreau P, Terpos E, et al. Multiple Myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. HemaSphere. 2021;5(2):e528. doi:10.1097/HS9.0000000000000528
[2] Xu Y, Zhang J, Zhou M, et al. Multiple myeloma complicated with myelodysplastic syndrome: case series and literature review. Ann Palliat Med. 2022;11(3):1104–1111. doi:10.21037/apm-21-2815
[3] Greenberg PL, Tuechler H, Schanz J, et al. Revised International Prognostic Scoring System for myelodysplastic syndromes (IPSS-R). Blood. 2012;120(12):2454–2465. doi:10.1182/blood-2012-03-420489
[4] List A, Kurtin S, Roe DJ, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456–1465. doi:10.1056/NEJMoa061292
[5] Krönke J, Fink EC, Hollenbach PW, et al. Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS. Nature. 2015;523(7559):183–188. doi:10.1038/nature14610