Patient case #5 - Acute Promyelocytic Leukemia

Description
A 9-year-old male presented to the emergency room with rapid onset pain, fever, cough, headache, shortness of breath, nausea, and vomiting.
Viral infection was suspected and rapid tests for RSV and influenza were ordered. However, over the next several hours, the child became increasingly ill and developed petechiae on his arms and legs.
CBC Results:
| Test | Result | Units |
|---|---|---|
| WBC | 2.1 | X109/L |
| HGB | 7.3 | g/dL |
| HCT | 22.0 | % |
| PLT | 6 | X109/L |
Cellavision DC-1 Differential:
| Cell class | % | x109/L |
|---|---|---|
| Neutrophils | 0.3 | <.01 |
| Lymphocytes | 28.7 | 0.60 |
| Monocytes | 0.3 | <.01 |
| *Blast + Promyelocytes | 70.1 | 1.47 |
| NRBCs | 1.2 / 100WBC | |
| *Abnormal promyelocytes are considered blast equivalents in blood and bone marrow differentials1, 2. |
||
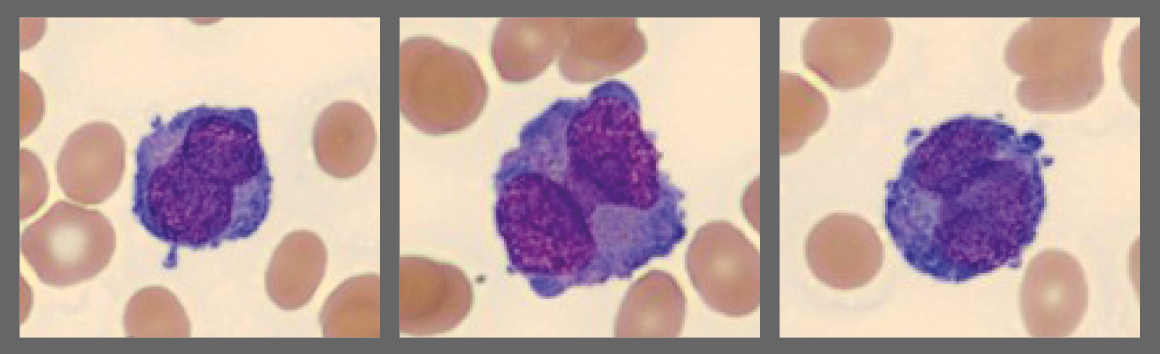
WBC differential showed a large population of abnormal cells with immature chromatin and bilobed, dumbbell, and other nuclear shapes. Some cells were agranular, resembling blasts while others contained dense reddish granulation and one or more Auer rods. The abnormal cells were identified as abnormal promyelocytes and the clinician was immediately notified.
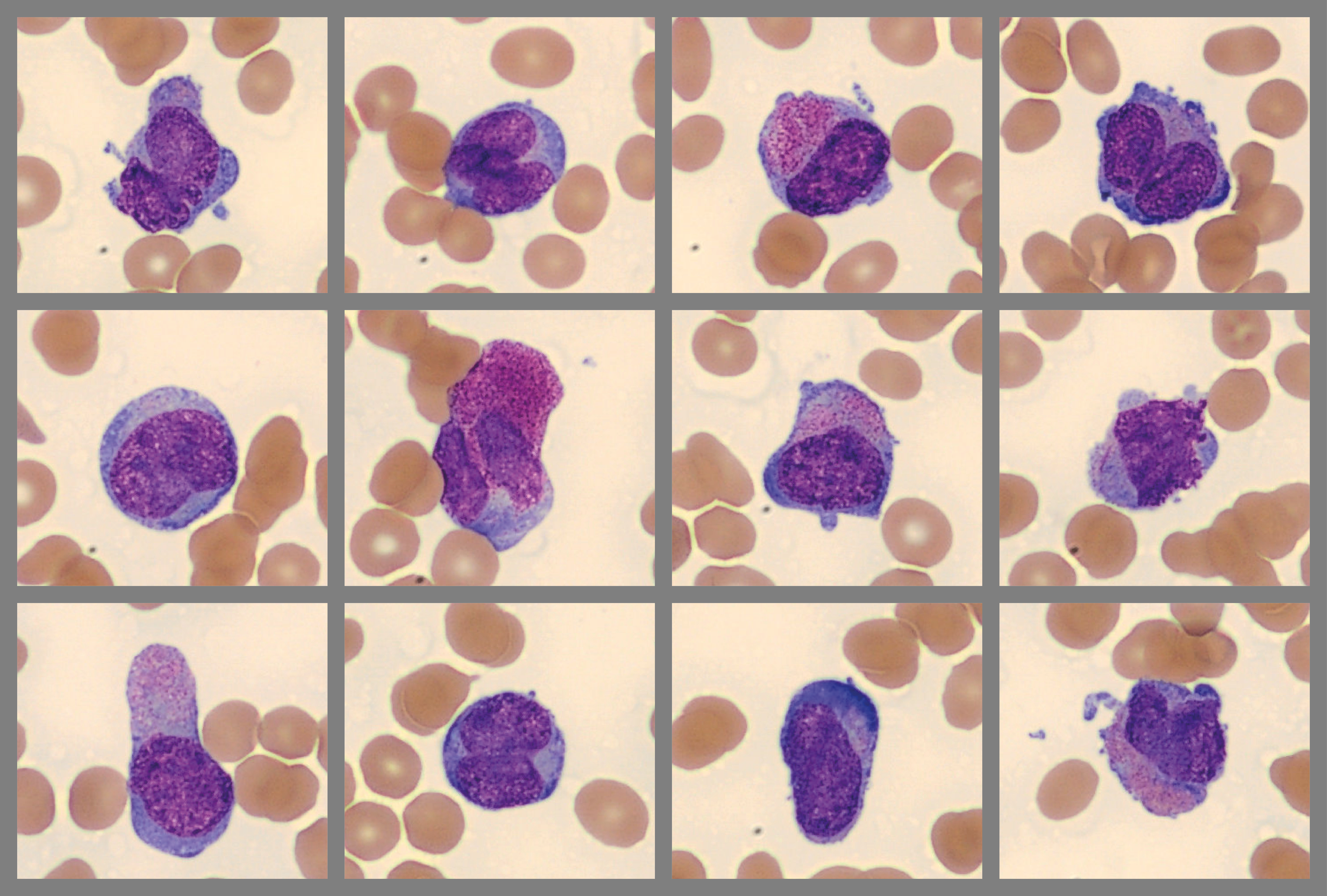
Abnormal promyelocytes, two with Auer rods:
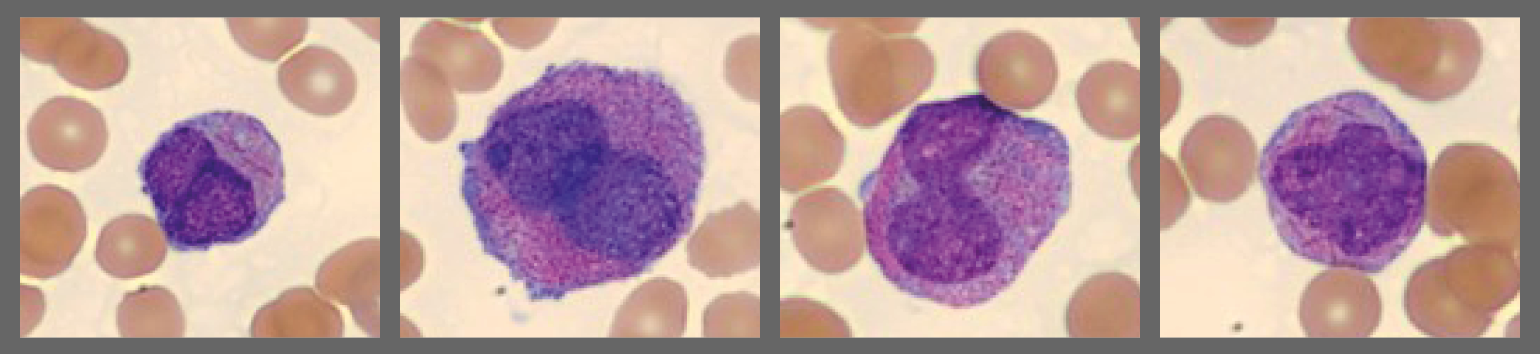
Hypogranular promyelocytes (These cells can resemble monocytes in other leukemias):
Morphology and clinical findings suggested acute promyelocytic leukemia (APL), and treatment was promptly initiated.
Diagnosis
Molecular testing was positive for PML::RARA, confirmed diagnosis of acute promyelocytic leukemia with PML::RARA fusion.
Discussion
APL represents 5 – 20% of acute myeloid leukemia (AML) cases; occurs at any age, but mostly 20–59 years old. A translocation between chromosomes 15 and 17, t(15;17) creates an abnormal gene PML::RARA which blocks promyelocyte maturation. Promyelocytes with distinctive morphology are seen in bone marrow and blood.
Two APL subtypes:
- Hypergranular APL (75% of cases): large promyelocytes with dense red-purple granulation and variable nuclear shapes/sizes, some cells have > 1 Auer rods. WBC count is usually low. The blood smear may show only a few abnormal promyelocytes. Digital morphology can be helpful in locating and presenting these cells to the user, especially in cases with low WBC count.
- Microgranular APL (25% of cases): promyelocytes have no or few granules and bilobed or “butterfly” nuclei. WBC count is often high.
As seen in this case, hypergranular and hypogranular promyelocytes can coexist in the same patient1.
Treatment/Prognosis
Without treatment, median survival is < 1 month due to hemorrhage. Thus, APL is a medical emergency and quick recognition of abnormal promyelocytes is key. Treatment decisions are based on detecting these cells in blood and/or bone marrow slides and clinical findings. Standard treatment is all-trans retinoic acid (ATRA) with arsenic trioxide or chemotherapy. ATRA induces maturation of promyelocytes to mature neutrophils. With ATRA, the long-term cure rate is > 90%1.
APL is a disease in which expertise in blood smear morphology can potentially be lifesaving.
Note: Feather edge analysis on Cellavision DC-1 found a large concentration of WBCs.
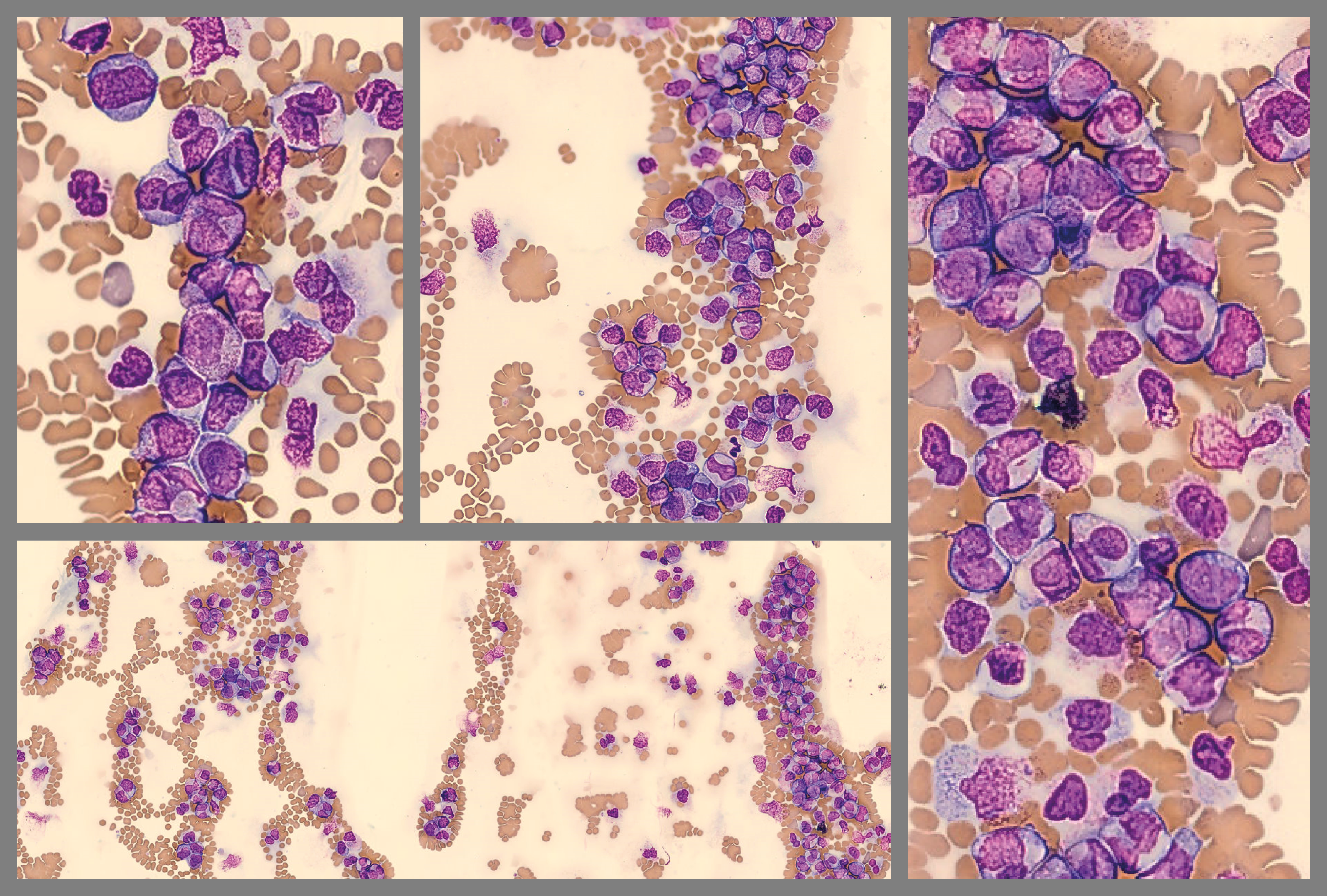
This problem is usually due to technical errors in slide preparation3. Larger cells are pushed to the end of the smear. Poor WBC distribution can affect the accuracy of differential counts; therefore, some labs require a new slide to be made.
___
1. WHO Classification of Tumours Editorial Board. Haematolymphoid tumours [Internet]. 5th ed. International Agency for Research on Cancer; 2024. Accessed 8/16/2024. https://tumourclassification.iarc.who.int/chapters/63.
2. Palmer L, Briggs C, McFadden S, et al. ICSH recommendations for the standardization of nomenclature and grading of peripheral blood cell morphological features. Int J Lab Hematol. Jun 2015;37(3):287-303. doi:10.1111/ijlh.12327
3. Butina MM. Body Fluid Analysis in the Hematology Laboratory. In: Keohane EM, ed. Rodak's Hematology : clinical principles and applications. 7th ed. Elsevier; 2025:chap E4. https://evolve.elsevier.com/Resources/167113_selfstudy_0001#/content/26804466904